Type: Compound
Vitamin: K
Name: Phylloquinone, Menaquinones,Phytomenadione (K1)
RDA:
Males: 70 µg
Females: 55 µg
Obtaining adequate amount is not a problem.
Importance- to Body:
Essential for formation of clotting proteins and some other proteins made by liver; as intermediate in electron transport chain, participates in oxidative phosphorylation in all body cells.
Distribution- in Body:
Most synthesized by coliform bacteria in large intestine. In a number of related compounds known as quinones; small amounts stored in liver; heat resistant; destroyed by acids, alkalis, light, oxidizing agents; activity antagonized by certain anticoagulants and antibiotics that interfere with synthetic activity of enteric bacteria.
Excess Effects:
None listed, (not stored in appreciable amounts).
Deficiency Effects:
Easy Bruising and Easy Bleeding (prolonged clotting time)
Food Sources:
Leafy Green Vegetables, Cabbage, Cauliflower, Pork Liver
Environmental/Geographic Sources:
None listed
Supplemental information:
| Vitamin K | |
|---|---|
| Drug class | |
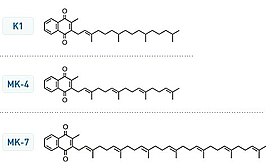
Vitamin K structures. MK-4 and MK-7 are both subtypes of K2.
|
|
| Class identifiers | |
| Use | Vitamin K deficiency, Warfarin overdose |
| ATC code | B02BA |
| Biological target | Gamma-glutamyl carboxylase |
| Clinical data | |
| Drugs.com | Medical Encyclopedia |
| External links | |
| MeSH | D014812 |
| In Wikidata | |
Vitamin K is a group of structurally similar, fat-soluble vitamins that the human body requires for complete synthesis of certain proteins that are prerequisites for blood coagulation (K from Koagulation, Danish for "coagulation") and which the body also needs for controlling binding of calcium in bones and other tissues. The vitamin K-related modification of the proteins allows them to bind calcium ions, which they cannot do otherwise. Without vitamin K, blood coagulation is seriously impaired, and uncontrolled bleeding occurs. Preliminary clinical research indicates that deficiency of vitamin K may weaken bones, potentially leading to osteoporosis, and may promote calcification of arteries and other soft tissues.
Chemically, the vitamin K family comprises 2-methyl-1,4-naphthoquinone (3-) derivatives. Vitamin K includes two natural vitamers: vitamin K1 and vitamin K2. Vitamin K2, in turn, consists of a number of related chemical subtypes, with differing lengths of carbon side chains made of isoprenoid groups of atoms.
Vitamin K1, also known as phylloquinone, is made by plants, and is found in highest amounts in green leafy vegetables because it is directly involved in photosynthesis. It may be thought of as the plant form of vitamin K. It is active as a vitamin in animals and performs the classic functions of vitamin K, including its activity in the production of blood-clotting proteins. Animals may also convert it to vitamin K2.
Bacteria in the gut flora can also convert K1 into vitamin K2 (menaquinone). In addition, bacteria typically lengthen the isoprenoid side chain of vitamin K2 to produce a range of vitamin K2 forms, most notably the MK-7 to MK-11 homologues of vitamin K2. All forms of K2 other than MK-4 can only be produced by bacteria, which use these forms in anaerobic respiration. The MK-7 and other bacterially derived forms of vitamin K2 exhibit vitamin K activity in animals, but MK-7's extra utility over MK-4, if any, is unclear and is a matter of investigation.
Because a synthetic form of vitamin K, vitamin K3 (menadione), may be toxic by interfering with the function of glutathione, it is no longer used to treat vitamin K deficiency.

