Type: Beta Hydroxy Acid
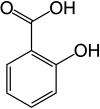
Name:Salicylic Acid
RDANone Listed
Importance- to Body:Skin, Anti-inflammatory, Exfoliant, Unclogs Pores, Oil Soluble (Penetrates into pores and oily regions of skin), Best for Blackheads and Whiteheads
Distribution- in Body:
Skin, Circulatory System, Digestive System
Excess Effects:
Irritation, Dryness and Flaking, Rashes, Peeling, flaking, Redness, Tenderness, Swelling, Itching
Deficiency Effects:
Dull Skin, Dry Skin, Whiteheads, Blackheads, Oily Skin, Acne
Food Sources:
Cucumbers, Alfalfa, Apricots, Artichokes, Eggplant, Mushrooms, Sauerkraut, Peppers, Olives, Lemons, Mangos, Passionfruit, Pomegranates, Strawberries, Blackberries, Blueberries, Pineapple, Grapes, Plums, Kiwi, Tomatoes, Nuts, Chicory, Herbs and Spices (all contain natural amounts of salicylates)
Environmental/Geographic Sources: Found in Willow Bark
Supplemental Information:
Found in: Face Cleansers, Serums, Creams, Peels, Moisturizers, Toners, Spot Treatments, Acne Kits, Soaps, Masks, Peel Pads, Some Foundations and Concealers
Sources:
O’Connor, Tracy. “Hydroxy Acids: What They Do and Which Ones Are Right for You.” Dermstore Blog, 11 Sept. 2017
Jacques, Renee. “Here’s Exactly What Salicylic Acid Does to Your Skin” Allure, Allure Magazine, 23 Oct. 2017
|
| |||

| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2-Hydroxybenzoic acid | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.000.648 | ||
| EC Number | 200-712-3 | ||
| KEGG | |||
PubChem CID
|
|||
| RTECS number | VO0525000 | ||
| UNII | |||
| |||
| |||
| Properties | |||
| C7H6O3 | |||
| Molar mass | 138.12 g·mol−1 | ||
| Appearance | Colorless to white crystals | ||
| Odor | Odorless | ||
| Density | 1.443 g/cm3 (20 °C) | ||
| Melting point | 158.6 °C (317.5 °F; 431.8 K) | ||
| Boiling point | 200 °C (392 °F; 473 K) decomposes 211 °C (412 °F; 484 K) at 20 mmHg | ||
| Sublimes at 76 °C | |||
| |||
| Solubility | Soluble in ether, CCl4, benzene, propanol, acetone, ethanol, oil of turpentine, toluene | ||
| Solubility in benzene |
| ||
| Solubility in chloroform |
| ||
| Solubility in methanol |
| ||
| Solubility in olive oil | 2.43 g/100 g (23 °C) | ||
| Solubility in acetone | 39.6 g/100 g (23 °C) | ||
| log P | 2.26 | ||
| Vapor pressure | 10.93 mPa | ||
| Acidity (pKa) |
| ||
| UV-vis (λmax) | 210 nm, 234 nm, 303 nm (4 mg % in ethanol) | ||
| −72.23·10−6 cm3/mol | |||
Refractive index (nD)
|
1.565 (20 °C) | ||
| Thermochemistry | |||
Std enthalpy of
formation (ΔfH |
−589.9 kJ/mol | ||
Std enthalpy of
combustion (ΔcH |
3.025 MJ/mol | ||
| Pharmacology | |||
| A01AD05 (WHO) B01AC06 (WHO) D01AE12 (WHO) N02BA01 (WHO) S01BC08 (WHO) | |||
| Hazards | |||
| Safety data sheet | MSDS | ||
| GHS pictograms |   | ||
| GHS signal word | Danger | ||
| H302, H318 | |||
| P280, P305+351+338 | |||
| Eye hazard | Severe irritation | ||
| Skin hazard | Mild irritation | ||
| NFPA 704 | |||
| Flash point | 157 °C (315 °F; 430 K) closed cup | ||
| 540 °C (1,004 °F; 813 K) | |||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
480 mg/kg (mice, oral) | ||
| Related compounds | |||
Related compounds
|
Methyl salicylate, Benzoic acid, Phenol, Aspirin, 4-Hydroxybenzoic acid, Magnesium salicylate, Choline salicylate, Bismuth subsalicylate, Sulfosalicylic acid | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Salicylic acid (from Latin salix, willow tree) is a lipophilic monohydroxybenzoic acid, a type of phenolic acid, and a beta hydroxy acid (BHA). It has the formula C7H6O3. This colorless crystalline organic acid is widely used in organic synthesis and functions as a plant hormone. It is derived from the metabolism of salicin. In addition to serving as an important active metabolite of aspirin (acetylsalicylic acid), which acts in part as a prodrug to salicylic acid, it is probably best known for its use as a key ingredient in topical anti-acne products. The salts and esters of salicylic acid are known as salicylates.
It is on the WHO Model List of Essential Medicines, the most important medications needed in a basic health system.