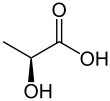
Type: Alpha Hydroxy Acid (AHA)
Name: Lactic Acid
RDA:None Listed
Importance- to body:
Exfoliant (sloughs off dead skin cells), Used for Sensitive and Dry Skin, Gentle, Hydrating, Less irritating than other AHAs, Brightens Skin
Distribution- in body:
Skin
Excess effects:
Irritation, Dryness, Rashes, Peeling, Redness, Tenderness, Swelling, Itching, Increased Sunburn
Deficiency effects:
Dull Skin, Dry Skin, Whiteheads, Blackheads
Food Sources:
Yogurt, Sourdough Bread, Pickled Vegetables, Wine, Salads and Dressings
Environmental/Geographic Sources:
Face Cleansers, Serums, Creams, Peels, Moisturizers
Supplemental information:
Sources::
Gromisch, Maryann. “What Foods Contain Lactic Acid?” LIVESTRONG.COM, Leaf Group, 3 Oct. 2017
Trench, Brooke Le Poer, and Kara Nesvig. “6 Things You Should Know Before Putting This Popular Acid on Your Skin” Allure, Allure Magazine, 23 Oct. 2017
|
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2-Hydroxypropanoic acid | |||
| Other names
Milk acid
| |||
| Identifiers | |||
| |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.017 | ||
| E number | E270 (preservatives) | ||
| UNII | |||
| |||
| |||
| Properties | |||
| C3H6O3 | |||
| Molar mass | 90.08 g·mol−1 | ||
| Melting point | 53°C | ||
| Boiling point | 122 °C (252 °F; 395 K) @ 15 mmHg | ||
| Acidity (pKa) | 3.86, 15.1 | ||
| Thermochemistry | |||
Std enthalpy of
combustion (ΔcH |
1361.9 kJ/mol, 325.5 kcal/mol, 15.1 kJ/g, 3.61 kcal/g | ||
| Pharmacology | |||
| G01AD01 (WHO) QP53AG02 (WHO) | |||
| Hazards | |||
| GHS pictograms |  | ||
| H315, H318 | |||
| P280, P305+351+338 | |||
| Related compounds | |||
Other anions
|
lactate | ||
Related carboxylic acids
|
acetic acid glycolic acid propionic acid 3-hydroxypropanoic acid malonic acid butyric acid hydroxybutyric acid | ||
Related compounds
|
1-propanol 2-propanol propionaldehyde acrolein sodium lactate | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Lactic acid is an organic compound with the formula CH3CH(OH)COOH. In its solid state, it is white and water-soluble. In its liquid state, it is colorless. It is produced both naturally and synthetically. With a hydroxyl group adjacent to the carboxyl group, lactic acid is classified as an alpha-hydroxy acid (AHA). In the form of its conjugate base called lactate, it plays a role in several biochemical processes.
In solution, it can ionize a proton from the carboxyl group, producing the lactate ion CH
3CH(OH)CO−
2. Compared to acetic acid, its pKa is 1 unit less, meaning lactic acid deprotonates ten times more easily than acetic acid does. This higher acidity is the consequence of the intramolecular hydrogen bonding between the α-hydroxyl and the carboxylate group. Lactic acid is chiral, consisting of two optical isomers. One is known as L-(+)-lactic acid or (S)-lactic acid and the other, its mirror image, is D-(−)-lactic acid or (R)-lactic acid. A mixture of the two in equal amounts is called DL-lactic acid, or racemic lactic acid. Lactic acid is hygroscopic. DL-lactic acid is miscible with water and with ethanol above its melting point which is around 17 or 18 °C. D-lactic acid and L-lactic acid have a higher melting point.
In animals, L-lactate is constantly produced from pyruvate via the enzyme lactate dehydrogenase (LDH) in a process of fermentation during normal metabolism and exercise. It does not increase in concentration until the rate of lactate production exceeds the rate of lactate removal, which is governed by a number of factors, including monocarboxylate transporters, concentration and isoform of LDH, and oxidative capacity of tissues. The concentration of blood lactate is usually 1–2 mM at rest, but can rise to over 20 mM during intense exertion and as high as 25 mM afterward. In addition to other biological roles, L-lactic acid is the primary endogenous agonist of hydroxycarboxylic acid receptor 1 (HCA1), which is a Gi/o-coupled G protein-coupled receptor (GPCR).
In industry, lactic acid fermentation is performed by lactic acid bacteria, which convert simple carbohydrates such as glucose, sucrose, or galactose to lactic acid. These bacteria can also grow in the mouth; the acid they produce is responsible for the tooth decay known as caries. In medicine, lactate is one of the main components of lactated Ringer's solution and Hartmann's solution. These intravenous fluids consist of sodium and potassium cations along with lactate and chloride anions in solution with distilled water, generally in concentrations isotonic with human blood. It is most commonly used for fluid resuscitation after blood loss due to trauma, surgery, or burns.