Type: Essential Amino Acid
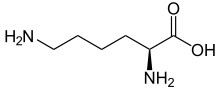
Name: Lysine (Lys, K), Chemical Formula C6H14N202
Importance- to body: Improves athletic performance, treats and prevents cold sores (herpes simplex virus), creates carnitine (carnitine converts fatty acids to energy), helps form collagen (hair, nails), helps body in the absorption of calcium, supports a healthy gut, reduces symptoms of diabetes, aids in healthy bones.
Distribution- in Body:
In the gut, Liver, Muscle Tissues.
Excess Effects:
Stomach Discomfort, Diarrhea, High Cholesterol, Gallstones.
Deficiency Effects:
Loss of Appetite, Mood Swings, Loss of Hair, Anemia, Stunted Growth.
Food Sources:
Meats (Beef, Lamb, Turkey, Chicken, Pork), Tuna, Shrimp, Soy Beans, Eggs, White Beans, Pumpkin Seeds, Beans, Cheeses, Eggs.
Environmental/Geographic Sources:
Supplemental Information:
Aids in the recovery of herpes virus, and also improves mood disorders (depression) and can treat anxiety. First identified by Dreschel in 1889.
Works Cited:
1. Uddin, Rae. “Signs & Symptoms of Lysine Deficiency.” LIVESTRONG.COM, Leaf Group, 3 Oct. 2017, www.livestrong.com/article/342821-signs-symptoms-of-lysine-deficiency/.
2. Edwards, Rebekah. “Top 10 Highest L-Lysine-Rich Foods (It Fights Herpes…and Maybe Even Cancer).” Dr. Axe, Dr. Axe, 15 June 2017, draxe.com/l-lysine-benefits/.
 L-lysine
|
|
| Names | |
|---|---|
| IUPAC name
(2S)-2,6-Diaminohexanoic acid (L-lysine) (2R)-2,6-Diaminohexanoic acid (D-lysine)
|
|
| Other names
Lysine, D-lysine, L-lysine, LYS, h-Lys-OH
|
|
| Identifiers | |
|
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.673 |
| KEGG | |
|
PubChem CID
|
|
| UNII | |
|
|
|
|
| Properties | |
| C6H14N2O2 | |
| Molar mass | 146.19 g·mol−1 |
| 1.5 kg/L | |
| Pharmacology | |
| B05XB03 (WHO) | |
| Supplementary data page | |
| Refractive index (n), Dielectric constant (εr), etc. |
|
|
Thermodynamic
data |
Phase behaviour solid–liquid–gas |
| UV, IR, NMR, MS | |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
| Infobox references | |
Lysine (symbol Lys or K) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH3+ form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a side chain lysyl ((CH2)4NH2), classifying it as a basic, charged (at physiological pH), aliphatic amino acid. It is encoded by the codons, AAA and AAG. Like almost all other amino acids, the α-carbon is chiral and lysine may refer to either enantiomer or a racemic mixture of both. For the purpose of this article, lysine will refer to the biologically active enantiomer L-lysine, where the α-carbon is in the S configuration.
The human body cannot synthesize lysine, so it is essential in humans and must be obtained from the diet. In organisms that synthesise lysine, it has two main biosynthetic pathways, the diaminopimelate and α-aminoadipate pathways, which employ different enzymes and substrates and are found in different organisms. Lysine catabolism occurs through one of several pathways, the most common of which is the saccharopine pathway.
Lysine plays several roles in humans, most importantly proteinogenesis, but also in the crosslinking of collagen polypeptides, uptake of essential mineral nutrients, and in the production of carnitine, which is key in fatty acid metabolism. Lysine is also often involved in histone modifications, and thus, impacts the epigenome. The ε-amino group often participates in hydrogen bonding and as a general base in catalysis. The ε-ammonium group (NH3+) is attached to the fourth carbon from the α-carbon, which is attached to the carboxyl (C=OOH) group.
Due to its importance in several biological processes, a lack of lysine can lead to several disease states including defective connective tissues, impaired fatty acid metabolism, anaemia, and systemic protein-energy deficiency. In contrast, an overabundance of lysine, caused by ineffective catabolism, can cause severe neurological issues.
Lysine was first isolated by the German biological chemist Ferdinand Heinrich Edmund Drechsel in 1889 from the protein casein in milk. He named it "lysin". In 1902, the German chemists Emil Fischer and Fritz Weigert determined lysine's chemical structure by synthesizing it.

