Type: Compound
Vitamin: C
Name: Ascorbic Acid,L-Ascorbic Acid
RDA: 60 mg (100 mg for those who smoke)
Importance- to Body:
Acts in hydroxylation reactions in formation of nearly all connective tissues; in conversion of tryptophan to serotonin; in conversion of cholesterol to bile salts; helps protect vitamins A and E and dietary fats from oxidation.
Distribution- in Body:
Simple 6-carbon crystalline compound derived from glucose; rapidly destroyed by heat, light, alkalis; about 1500 mg is stored in body, particularly in adrenal gland, retina, intestine, pituitary; when tissues are saturated.
Excess Effects:
Generally large doses may cause Gastrointestinal Discomfort, Headache, Trouble Sleeping, and Flushing of the Skin. Results of mega doses (10 or more times RDA); Enhanced Mobilization of Bone Minerals and Blood Coagulation; Exacerbation of Gout; Kidney Stone Formation
Deficiency Effects:
Defective formation of intercellular cement; Fleeting Joint Pains, Poor Tooth and Bone Growth; Poor Wound Healing, Increased Susceptibility to Infection; Extreme deficit causes Scurvy (more generally known as Periodontal Disease today).
Food Sources:
Fruit, Tomatoes, Cantaloupe, Strawberries, Vegetables, Potatoes, Leafy Greens
Environmental/Geographic Sources:
None listed
Supplemental information:
 |
|
 |
|
| Clinical data | |
|---|---|
| Synonyms | L-ascorbic acid, ascorbic acid, ascorbate |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682583 |
| Pregnancy category |
|
| Routes of administration |
By mouth, IM, IV, subQ |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | rapid & complete |
| Protein binding | negligible |
| Biological half-life | varies according to plasma concentration |
| Excretion | kidney |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| NIAID ChemDB | |
| E number | E300 (antioxidants, ...) |
| ECHA InfoCard | 100.000.061 |
| Chemical and physical data | |
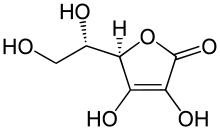
| Formula | C6H8O6 |
| Molar mass | 176.12 g/mol |
| 3D model (JSmol) | |
| Density | 1.694 g/cm3 |
| Melting point | 190 °C (374 °F) |
| Boiling point | 553 °C (1,027 °F) |
|
|
|
|
| (verify) | |
Vitamin C, also known as ascorbic acid and L-ascorbic acid, is a vitamin found in food and used as a dietary supplement. The disease scurvy is prevented and treated with vitamin C-containing foods or dietary supplements. Evidence does not support use in the general population for the prevention of the common cold. There is, however, some evidence that regular use may shorten the length of colds. It is unclear if supplementation affects the risk of cancer, cardiovascular disease, or dementia. It may be taken by mouth or by injection.
Vitamin C is generally well tolerated. Large doses may cause gastrointestinal discomfort, headache, trouble sleeping, and flushing of the skin. Normal doses are safe during pregnancy. The United States Institute of Medicine recommends against taking large doses.
Vitamin C is an essential nutrient involved in the repair of tissue and the enzymatic production of certain neurotransmitters. It is required for the functioning of several enzymes and is important for immune system function. It also functions as an antioxidant. Foods containing vitamin C include citrus fruits, broccoli, Brussels sprouts, raw bell peppers, and strawberries. Prolonged storage or cooking may reduce vitamin C content in foods.
Vitamin C was discovered in 1912, isolated in 1928, and in 1933 was the first vitamin to be chemically produced. It is on the World Health Organization Model List of Essential Medicines, the most effective and safe medicines needed in a health system. Vitamin C is available as a generic medication and over-the-counter drug. In 2015, the wholesale cost in the developing world was less than US$0.01 per tablet. Partly for its discovery, Albert Szent-Györgyi and Walter Norman Haworth were awarded 1937 Nobel Prizes in Physiology and Medicine and Chemistry, respectively.

